What the curriculum thinks you need to know:
PC_BK_21 Physics of vapours
PC_BK_33 Vapour pressure: saturated vapour pressure
PC_BK_34 Vaporisation: process of vaporisation
PC_BK_35 Vaporisers: principles, including plenum and draw-over, temperature compensation, concentration
What you need to know (The theory):
Vapour
A substance in the gaseous phase, below its critical temperature.
Remember critical temperature is the temperature above which a gas cannot be liquefied, no matter the pressure applied to it.
Vapour Pressure
The proportion of atmospheric pressure (partial pressure) attributable to a vapour
If we put a liquid in a closed container in contact with a volume of gas, some of that liquid will vapourise, or move to the gaseous phase. This is due to the movement and kinetic energy of the liquid causing molecules to ‘jump’ from the surface. This gives liquids their smell, so next time you crack open that bottle of beer, you’re smelling the beer vapour…

How a Vapour pressure is made…
That vapour which is produced will exert a small, but measurable pressure in the closed container. This is the saturated vapour pressure (SVP).
Saturated vapour pressure
The partial pressure generated by a vapour in equilibrium with its liquid form at standard temperature and pressure (STP)
The key word here is equilibrium. SVP is normally stated at standard temperature and pressure, so it stands to reason that the SVP is altered by the surrounding temperature and pressure. An increase in temperature will increase the SVP as more energy is available, this becomes important later on…
Volatile anaesthetic agents
Water produces a vapour above it, but the SVP of water is very low (2.3kPa at 20oC). All of the agents used in anaesthesia are termed ‘volatile’, not because they can blow up (although some of the old ones did, Cyclopropane being the classic example), but as they readily produce a high vapour pressure.
| Agent | SVP (at 20 oC) | Boiling Point (oC) |
|---|---|---|
| Sevoflurane | 22 | 58.6 |
| Isoflurane | 33 | 48.5 |
| Desflurane | 88 | 22.8 |
| Halothane | 32.5 | 50.2 |
| Enflurane | 22.9 | 56.5 |
Notice here that the SVPs of all the commonly used agents today are the same double figures (e.g. 22, 33, 88) It makes it nice and easy to remember these for MCQs that way. Notice also the boiling point of desflurane, that’s important! (see below why…)
Also notice how the boiling points are almost inverse to their SVP. Agents with a low SVP (less volatile!) have a higher boiling point, so need more ‘help’ to boil. Agents with a high SVP (very volatile) need little to no help in boiling.
What you need to know (How it works in practice):
Vapourisers
Note: The explanations below are slightly simplified to make them understandable. The exact ins and outs are slightly different and more complex. This is more than enough understanding to pass the exam!
Why bother with a vapouriser? Well first of all, if we just put a bit of agent in our breathing system it would generate its SVP in concentration, so for sevoflurane for example it would generate 22kPa. Now 22kPa out of 101.32kPa atmospheric pressure is a lot! This is 21% sevoflurane concentration, so getting on for 10 MAC, A good way to kill a patient quickly. Secondly, there would be no way to control the concentration of agent.
There are a few types of vapouriser that you need to know about, We’ll start with the simple Boyle’s/Plenum type vapouriser…
Lets Build a vapouriser…
People get all flustered about vapourisers and overcomplicate it, but if we keep in simple and build it up bit by bit it gets simpler… (Remember in a VIVA situation, keep your diagrams simple to begin with! You can always add things to the diagram if asked about it. Building it up bit by bit allows you to talk through it as you’re drawing, making more efficient usage of your viva time!)
First, lets get a pot of anaesthetic agent. Lets use sevoflurane as it smells nicer. Now the pot will have a layer of sevoflurane liquid covered by gas containing the SVP of sevoflurane vapour (eg 22 kPa out of 101.32 kPa atmospheric pressure)

Basic Vapouriser ‘Pot’
Now lets add a gas flow to get that anaesthetic vapour into… The resultant gas flow will now have the SVP of sevoflurane in it, assuming the flow is slow enough to let it equilibriate. This is 22 kPa, or approx. 21% concentration. Way, way too much to use in practice.

Basic ‘Pot’ Vapouriser with FGF.
So lets lower this concentration down. If we split the fresh gas flow (FGF) and only allow a proportion to go into the vapourising chamber and making some gas bypass it, we can lower the concentration of the resultant flow. For instance, here if we send 50% into the vapourising chamber and 50% through the bypass channel we can half the resultant concentration of sevoflurane in the gas flow. This is because we only saturate half the gas flow with sevoflurane. Our resultant concentration is then the SVP x 50% (22 x 0.5 = 11) or 11kPa.

Basic Vapouriser with Bypass channel to reduce the volatile concentration
If we allow half the FGF into the vapourising chamber, our resultant FGF will have half the SVP of our volatile agent in it. We can vary the amount of agent entering the chamber to then change the resultant concentration of the gas flow. This is known as adjusting the ‘splitting ratio’.
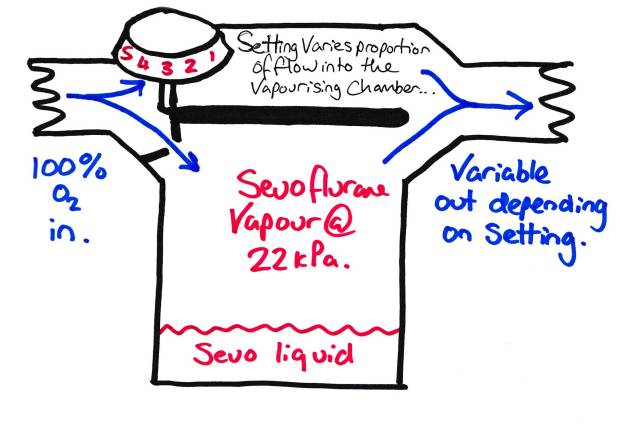
Vapouriser with Variable Bypass – This is basically what is on your anaesthetic machine.
This is what that big knob on the top of the vapouriser does, changes the proportion of the FGF that enters the vapourising chamber. So if we have 10% entering the vapourising chamber, the resultant FGF will have ~10% of the SVP in it. So for sevoflurane this would be about 2.2kPa or ~2.2% or ~ 1 MAC. Simple.
Problems with vapourisers
High Flow rates
If we have higher flow rates through the vapouriser, there isn’t much time for the gas flowing to equalibrilate and ‘pick up’ the vapour. There are a few ways to help with this:
- Wicks – These increase the surface area that the gas has to ‘pick up’ vapour from, making it easier to maintain concentration with high flow rates.
- Baffles – These make the FGF come in repeated contact with the vapourising surface, increasing the uptake of agent and ensuring a constant uptake with high flows.
- Bubbles – Bubbling the FGF through the anaesthetic agent drastically increases the surface area for uptake making it easier to keep constant concentrations with high flow rates.

Methods of ensuring FGF saturation with high flow rates.
Evaporative Cooling
When a volatile anaesthetic vapourises, an amount of energy is required to allow the change of phase (e.g. liquid to gaseous). This is the latent heat of vapourisation. This is defined as the energy required to change one kg of liquid to gas.
Now the problem with supplying this energy is that it cools the surrounding liquid. Now as we said earlier, this causes a change in SVP of the agent, reducing it in this case. This will lead to a lowered concentration of agent being supplied and your patient waking up or complaining of awareness. This effect is increased the higher the flows as more latent heat of vapourisation is needed. So how do we stop this?:
- Heat sinks – This is why your vapouriser weighs a tonne! The vapourising chamber is surrounded by a big hunk of metal. This has a high specific heat capacity and conducts heat to the vapour well, so minimises changes of temperature in the vapourising chamber. It transfers heat to the vapour, so appears cold. This is why your typical vapouriser is cool, and cools down when you have high flow rates.
- Bimetallic strips and bellows – These are simple devices that change the splitting ratio when the temperature changes. When the temperature cools, they move to allow more gas flow to enter the vapourising chamber keeping the concentration of agent in the FGF fairly constant. Bimetallic strips are strips made of two metals, each of which expand at different rates when the temperature rises. This causes the tip of the strip to move, opening up the entrance of the vapourising chamber. Liquid bellows work in the same way, as the liquid inside them cools, they contract causing the opening of the chamber to open, increasing the splitting ratio and increasing the ‘pickup’ of agent.

Methods of compensating for evaporative cooling of volatile agents.
Its important to note that all these things need to be calibrated to the individual anaesthetic agent. All the agents have different SVPs and their SVPs change at different rates with temperature. So all anaesthetic agents must be put in a vapouriser designed for that agent. Fairly obvious, but worth saying! **
Desflurane
Desflurane is special. Why? Look at the table further up. Notice anything interesting? That boiling point is an awful lot lower than the other agents, and very close to room temperature (well, maybe not in an orthopaedic theatre, but hey). Why is this important? Well when we get close to our boiling point, small changes in temperature cause large changes in SVP, making it difficult to compensate for in a plenum type vapouriser. We also have the risk of the agent boiling en mass leading to masses of agent going to the patient. Remember when an agent boils, its SVP equals atmospheric pressure. 101kPa of Desflurane will give you about 15 MAC, Ouch.
So we need a specific vapouriser.
First, we heat up the desflurane to a temperature above which we are unlikely to get to. In this case 39oC. Why? Because its easier to heat something to a constant temperature than it is to cool to a constant temperature. As this is over its boiling point all the desflurane is in gaseous form. The SVP of the desflurane at this temperature is approx. 200kPa.
The resultant vapour is then injected into the fresh gas flow directly, like a fuel injector in a car.
The amount injected is controlled by microprocessor. The processor analyses the amount of FGF, and the concentration dialled up on the vapouriser. It them calculates the amount of agent it needs to inject into the FGF to achieve this concentration.
DIVA type vaporisers
Note: The college haven’t asked about these specifically yet, but as they become more common, they may do!
Have you used one of the modern anaesthetic machines that controls the end tidal concentration of volatile agent for you? These machines (such as the Maquet Flow-I and similar) use a slightly different principle. Ever wondered why the vapourisers ‘click’ and why those clicks get faster as you have higher FGF rates?
These vapourisers have a heated vapourisation chamber into which small amounts of agent in liquid form are injected (this is the audible click). The heat causes these agents to instantly vapourise and this is then passed into the fresh gas flow. As the FGF and concentration of required agent increase, all that happens is these injections of agent become more frequent (and hence the clicks become more regular). These vapourisers all work in the same way regardless of agent, but are calibrated slightly differently for each agent.
Random Exam factoids (i.e. the things the college like asking):
- ** As halothane and isoflurane (and also enflurane and Sevoflurane) have very similar boiling points and SVPs, they theoretically could be used in the same vapouriser (not at the same time!!) with some degree of accuracy. Isoflurane and enflurane are structural isomers, but, they have VERY different SVPs and boiling points so cannot be used in the same vapouriser.
© Sam Beckett and Physics4FRCA, 2017. Unauthorized use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited.

What a simple and effective way of explaining!
Simply outstanding!
LikeLike
thanks!!!!
LikeLike
amazing…
LikeLike